Wat is de PROM-wijzer?
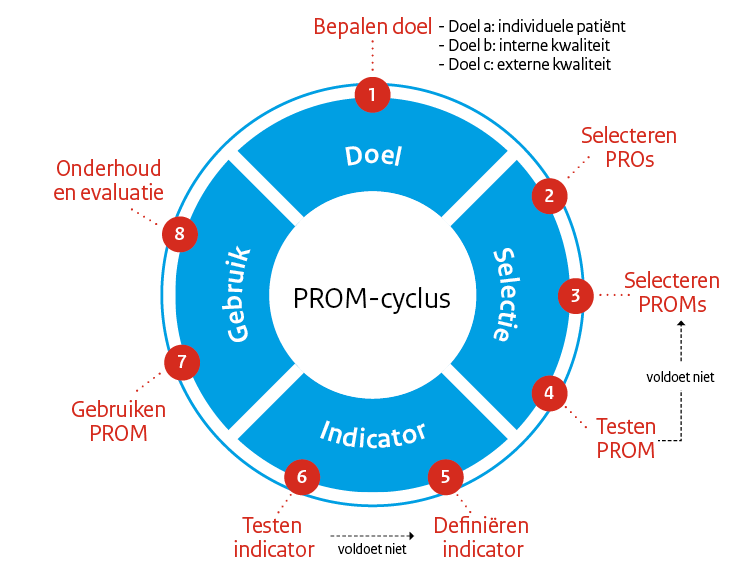
De PROM-wijzer maakt onderdeel uit van de PROM-toolbox en gaat over de oriëntatie en voorbereiding op het gebruik van PROMs. De PROM-wijzer komt qua volgordelijkheid dus vóór de PROM-cyclus, die is namelijk bedoeld voor de selectie en toepassing van PROMs in de gezondheidszorg.